Oxygen Mask Goes Odorless with GEON Flexible Vinyl
Expanding Into New Markets By Reducing Odor Without Performance Tradeoffs.
Challenge
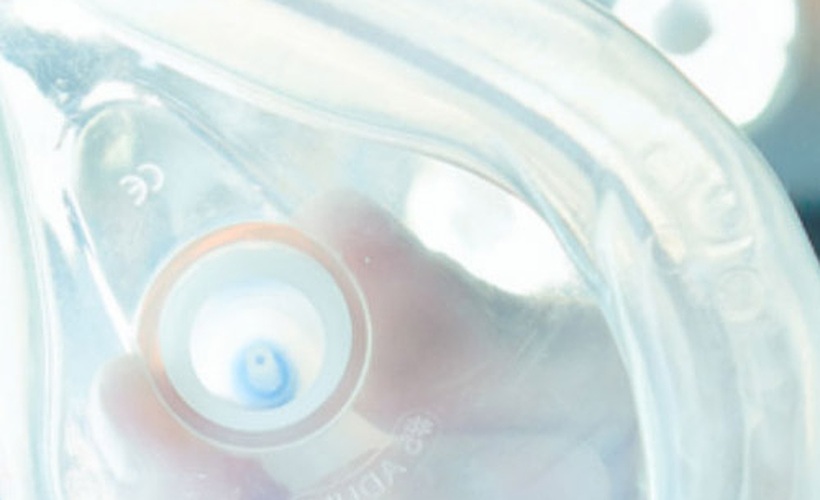
LIFE® Corporation is a U.S. Food and Drug Administration (FDA) registered manufacturer of medical devices specializing in CPR administration equipment and portable emergency oxygen based in Milwaukee, Wisconsin. Although LIFE Corporation had been manufacturing CPR masks for 25 years, when the company expanded into the Japanese market, a problem arose. LIFE Corporation discovered that the Japanese are exceptionally sensitive to the odors associated with their current vinyl material. In order for LIFE Corporation to be successful in Japan, LIFE Corporation needed a vinyl product offering minimal odor combined with high clarity and gloss, which would also meet regulatory approval for medical applications.
Solutions
After unsuccessful experimentation with many competitive materials, LIFE Corporation found GEON and trialed GEON Regulated XV1197 vinyl, which features high gloss, flexibility and excellent clarity.
According to LIFE’s president, John Kirchgeorg, “We tried many different suppliers over the years and we finally found GEON Regulated vinyl, which produces consistent clarity and flexibility without any detectable effluent odor, a necessity for our LIFE CPR masks used in all our LIFE OxygenPac emergency oxygen products.”
GEON’s Regulated XV1197 uses all FDA- compliant raw materials and passes United States Pharmacopeia (USP) Class VI testing, giving medical device manufacturers increased confidence that their end product will pass all applicable FDA qualification requirements.
The GEON material is biocompatible, which ensures the safety of both the victim and the first responder.
GEON Regulated XV1197 also enhances the performance of the masks: high clarity enables first responders to observe the victim’s condition (breathing, choking, etc.) and ensures correct positioning, while flexibility creates a firm seal around the victim’s face and forms a barrier protecting the first responder from direct contact with fluids from the patient.
Impact
GEON Regulated XV1197, with its minimal odor formulation, made a critical difference for LIFE Corporation by resolving a long-standing problem that was preventing the company from achieving its business goals.
The odorless formulation allows for broader market opportunities, such as in other Asian markets where odorless is the consumer preference. This product met LIFE’s customers’ need and enhanced their corporate reputation as a problem solver. The product also became more cost-effective because it is sterilizable with ethylene oxide (EtO) and reusable.
GEON Regulated PVC compounds also enabled LIFE Corporation to meet all of its performance requirements pertaining to clarity, flexibility, and regulatory compliance. The breadth of GEON’s offerings for the medical device industry made it easy to find a material with the precise properties for this unique application.
Kirchgeorg concluded, “The unique characteristics of GEON Regulated vinyl allowed LIFE Corporation to market our products worldwide and maintain our reputation for preferred, superior products.”


